Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium
DOI:
https://doi.org/10.3126/arj.v2i01.40738Keywords:
Alkaloid, extraction, weight loss, adsorption isotherm, thermodynamicsAbstract
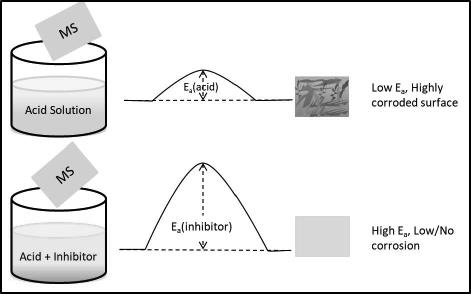
Alkaloids as green inhibitors were extracted from three different plants Rhynchostylis retusa, Artimesia vulgaris,and Solanum tuberosum. Weight loss measurement in mild steel has been carried out in the presence and absence of green inhibitors individually in an acidic medium. Weight loss measurements at different temperatures are used to calculate thermodynamic parameters. The weight loss measurements at different concentrations are used to find adsorption isotherm and found that it obeys Langmuir adsorption isotherm with R2 values 1, 1, 0.996 for three inhibitors. Activation energy, enthalpy, and entropy of the three inhibitors have been calculated. It is found that the value of all these parameters increased in the addition of inhibitors. The free energy of the system is calculated and found (-17.46 kJ mol-1) indicating that the adsorption process is spontaneous and there is physical adsorption at the MS-Inhibitor interface.